Stem Cells
Our Program is pursuing stem cell-based medicinals in clinical trials, building on our innovative basic research program in HSCs, iPSCs and the first ever completed clinical trial of an iPSC-derived cell therapy anywhere in the world (senior author ASH Dec 2018, Bloor et al Nature Medicine 2020). In collaboration with Cynata Therapeutics (Melbourne), in a multi-centre, open-label, dose escalation Phase I trial (NCT0923375) – allogeneic iPSC-derived MSCs were safe and well-tolerated in 15 patients with acute steroid-resistant graft versus host disease (GvHD). Based on our promising results, Cynata will expand clinical trials and translational research in partnership with academic sites including the university of Sydney in the following areas: 1) Phase I clinical trial of CymerusTM MSCs for treating diabetic wounds; 2) Develop iPSC-derived MSC therapy to minimise cytokine release syndrome after CAR-T therapy; 3) iPSC-derived MSCs for Phase II clinical trial in critical limb ischaemia.
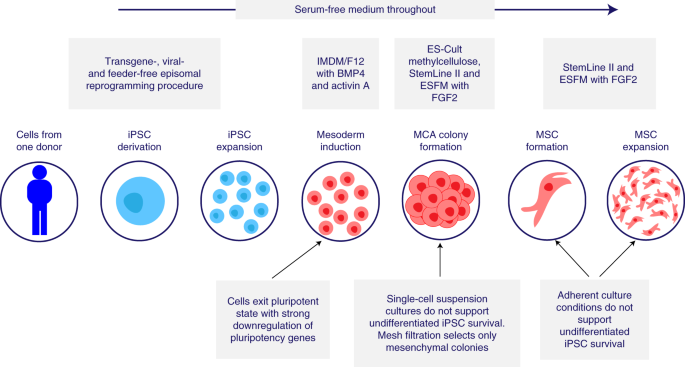
Overview of the CYP-001 manufacturing process
Our Program is importing the current manual GMP processes into automated, closed, industrial-scales. We will develop new tools in collaboration with biopharma to reduce the cost/duration to manufacture therapeutic lots and refine logistics/supply chains.
Find out more: Hussein et al, Nature 2014; Bloor et al Nature Medicine 2020